Collaborative researches with experimental researchers
We actively conduct collaborative researches with experimental researchers.
Functional mechanism of an iron-phytosiderophore transporter
An iron-phytosiderophore transporter, Yellow Stripe 1 (YS1) uptakes Fe3+ in complex with phytosiderophores (mugineic acids) and thereby plays an important role in absorbing poorly soluble iron through roots. The tertiary structures of YS1 alone and in complex with one of mugineic acids, deoxymugineic acid (DMA) (Fig. 1) binding Fe3+ (Fe(III)-DMA) were determined by cryogenic electron microscopy (cryo-EM). YS1 is a symporter that transports Fe(III)-DMA together with H+ from extracellular region to cytosol. In this study, we determined the protonation state of the cryo-EM structure and predicted the protonation sites involved in the transport. YS1 is comprised of the core domain and the scaffold domain. The transport of Fe(III)-DMA occurs via an elevator-like mechanism, in which the core domain moves with respect to the scaffold domain. We considered that some of the three acidic residues (D446, D490, and D494) located in the transmembrane regions of the core domain were involved in the transport (Fig. 2). We therefore conducted 500-ns MD simulations for YS1 with all the possible combinations of the protonation states of the three residues. Fe(III)-DMA was stably bound to the protein when only D494 was protonated, indicating that the cryo-EM structure is in this protonation state. When all the three residues were protonated, TM6 of the scaffold domain was detached from the core domain. This result suggests that the additional protonations of D446 and D490 would induce the movement of the core domain (Fig. 3). This study was conducted in collaboration with Dr. Yamagata’s group at RIKEN.
Yamagata et al., Nat. Commun. 13, 7180 (2022).
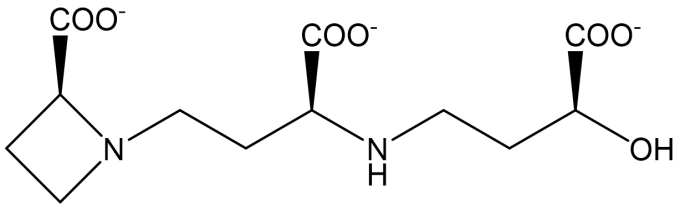
Fig. 1: The structure of deoxymugineic acid (DMA).
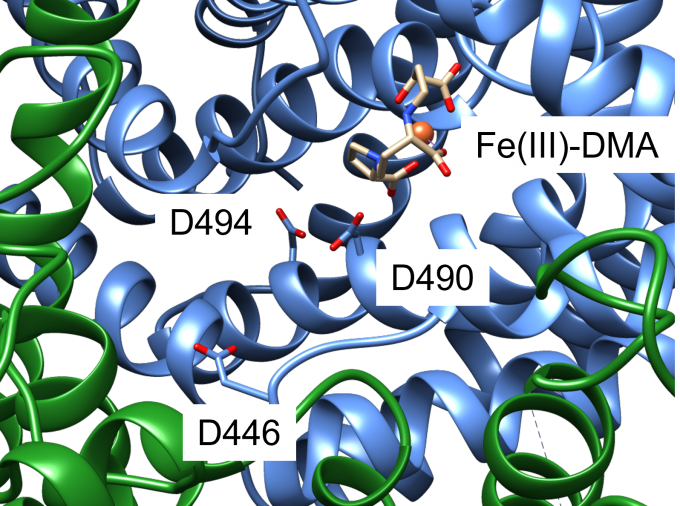
Fig. 2: A close-up view of the Fe(III)-DMA binding site of YS1. Core domain and scaffold domain are shown with blue and green ribbons, respectively. D446, D490, and D494 are shown with a stick representation. Fe3+ ion is shown with a sphere and DMA is shown with a stick representation.
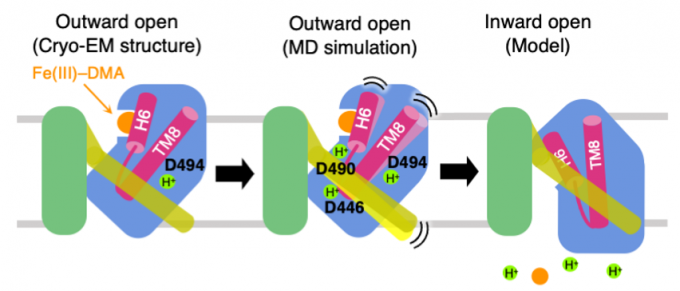
Fig. 3: Proposed mechanism of Fe(III)-DMA/H+ symport. The cryo-EM structure adopts the ourward-open conformation and stably binds Fe(III)-DMA with D494 protonated (left). When D446 and D490 are protonated in addition to D494, TM6 colored yellow is detached from the core domain colored blue (middle). Transition to the inward-open conformation occurs and Fe(III)-DMA and protons are released to the cytosol (right).
